Here's the dilemma with hydrogen: fueling your car with the stuff is faster than charging an EV, but making and distributing it is i...
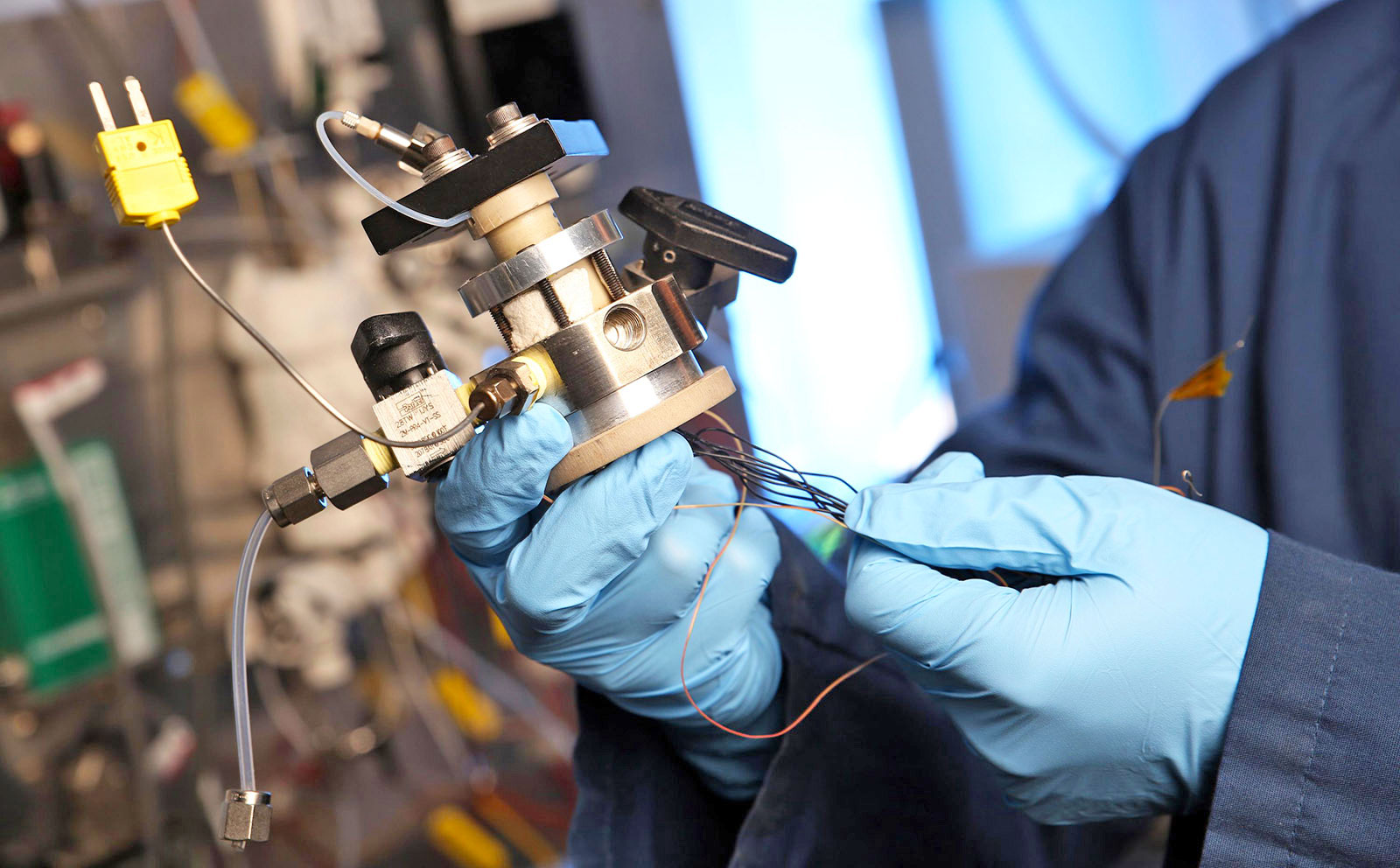
Here's the dilemma with hydrogen: fueling your car with the stuff is faster than charging an EV, but making and distributing it is inefficient and polluting. A team from the Georgia Institute of Technology has created a four-stroke "engine" that converts natural gas (methane) into hydrogen from just about anywhere, while capturing the CO2. It could one day hook up to your natural gas line, letting you fuel your car from home in a non-polluting way like you can with an EV -- pleasing both green tech boosters and oil companies.
The Georgia Tech team's device is called the CO2/H2 active membrane piston (CHAMP) reactor. Much like with a four-stroke engine, methane and steam are drawn into a cylinder when the piston goes down, as shown in the diagram below. It then rises and compresses the mixture to a temperature of around 400 degrees Celcius (752 F).
That causes a catalytic reaction, with no spark or explosion needed, that forms hydrogen gas (H2) and carbon dioxide (CO2). The H2 is absorbed by a membrane and exits the reaction chamber, while the CO2 is "adsorbed" in a so-called sorbent bed, where it combines with a catayst. A further cycle pulls the CO2 gas back into the cylinder and expels it in a concentrated form, where it can more easily be captured and stored.

Here's the dilemma with hydrogen: fueling your car with the stuff is faster than charging an EV, but making and distributing it is inefficient and polluting. A team from the Georgia Institute of Technology has created a four-stroke "engine" that converts natural gas (methane) into hydrogen from just about anywhere, while capturing the CO2. It could one day hook up to your natural gas line, letting you fuel your car from home in a non-polluting way like you can with an EV -- pleasing both green tech boosters and oil companies.
The Georgia Tech team's device is called the CO2/H2 active membrane piston (CHAMP) reactor. Much like with a four-stroke engine, methane and steam are drawn into a cylinder when the piston goes down, as shown in the diagram below. It then rises and compresses the mixture to a temperature of around 400 degrees Celcius (752 F).
That causes a catalytic reaction, with no spark or explosion needed, that forms hydrogen gas (H2) and carbon dioxide (CO2). The H2 is absorbed by a membrane and exits the reaction chamber, while the CO2 is "adsorbed" in a so-called sorbent bed, where it combines with a catayst. A further cycle pulls the CO2 gas back into the cylinder and expels it in a concentrated form, where it can more easily be captured and stored.





COMMENTS